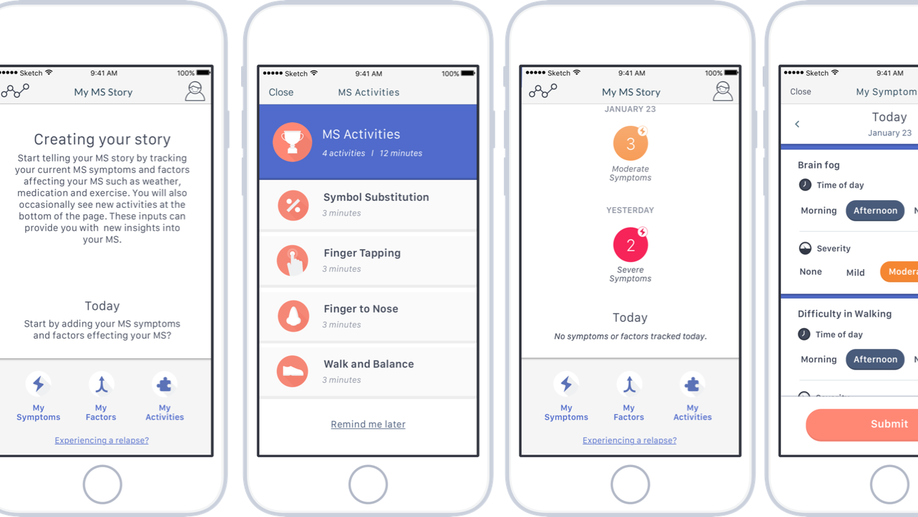
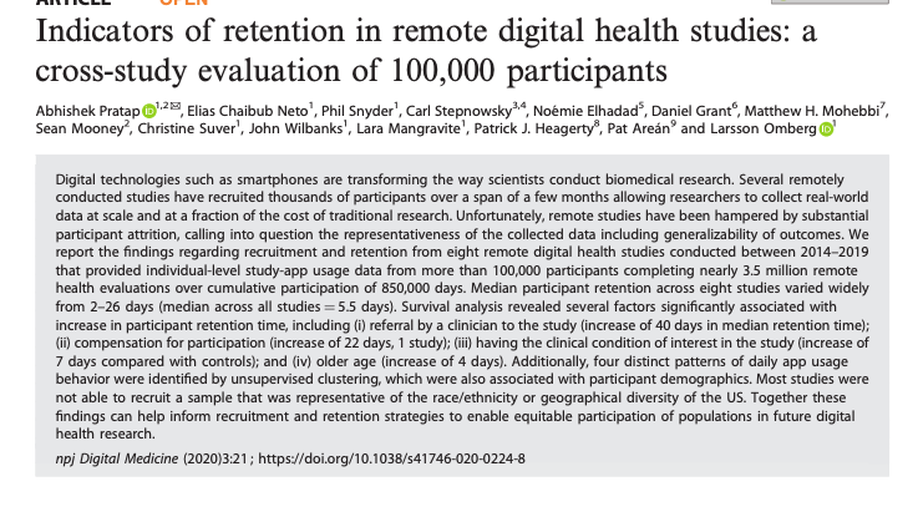
Abhi Pratap
Biography
Over 10 years of experience in biomedical informatics research. I have led several real-world digital health studies for assessing personalized lived experiences in individuals with neurological and psychiatric diseases.
My research is focused on development and validation of fit-for-purpose & contextual real-world digital endpoints for neurological and mental health disorders. I am also interested in understanding and addressing differential community participation in mental health research that may introduce significant biases in health data gathering in an uncontrolled “real world” settings. I believe in order to improve population-level outcomes in mental health we need to learn locally about what kind of health assessments and mediation mechanisms work for whom, when, and for how long?

I collaborate with an amazingly diverse stakeholders in developing decentralized studies. These include but not limited to communities with lived experience, clinicians, patient advocates, scientists, health communication specialists, data scientists , engineers and governance experts.
Interests
- Mental Health & Neruoscience
- Digital Health
- Decentralized Trials
- Stats/ML/AI
Education
PhD Biomedical Informatics (Digital Health)
University of Washington
MS Engineering Management (Computer Science)
University of Maryland, Baltimore County
Bachelors in Technology, Bioinformatics
VIT University